Gender clinics across Canada are participating in the largest international study of youth referred for hormones and puberty blockers. The Canadian study is funded with a grant from the Canadian Institutes of Health Research (CIHR). The study will be collecting data over a 2 year period on approximately 300 Canadian youth. Youth must be under 16 years of age upon enrollment to participate.

Goals of the Study
The Trans Youth Can! website declares that the study will be looking at “medical, social and family outcomes” and their goal is to produce “long overdue data on the health and well-being of trans youth who are referred for hormones and puberty blockers”.
The goals of the project, according to Dr Greta Bauer, Principle Investigator and Dr Margaret Lawson, Pediatric Endocrinologist and Co-principle Investigator, as outlined in a submission on Research Gate are as follows:
As a result of the research, we will know much more about the youth who are referred for gender issues and their families, including their previous care, gender identities, family dynamics, social stressors, and factors that affect their health and well-being. We will understand more about medical and mental health outcomes for youth undergoing puberty suppression and other medical interventions, and identify factors that may result in better outcomes, for example age at which treatment is started, type of treatment, or level of parental support. We will also learn about treatment side effects. We will know more about gender and sex development among these youth, and how their physical, mental and social health develops. In addition, we will study how family functioning and parent/caregiver’s well-being may change over time. We expect our study will result in changes to medical care, social services, and youth and family decision-making.”
Greta Bauer, PhD and Dr Margaret Lawson – Research Gate Goals
How did this study pass the ethics review process?
The goals outlined above and the website messaging include discussion of “outcomes” and treatment side effects including measurements of bone density. This makes the study sound like a treatment study on the health outcomes of youth being treated with puberty blockers and cross-sex hormones. We confirmed with Trans Youth Can!, however, that the study is an observational cohort.
We are an observational cohort study, not a treatment study, so there are no study drugs given or even study procedures (e.g. bloodwork) done as part of the study; we are gathering data from the youth and families for two years as they undergo the same care they would have received had they not been in the study.
Greta Bauer, PhD – Principal Investigator
Observational studies rely on survey data collected from patients at the gender clinics and are optional. Depending on the participation rate in the surveys, the data will be helpful to describe the population of youth undertaking medical interventions to affirm their gender identity.
The research ethics board approvals that this study has passed, therefore, are limited to approving a study of “observations” collected via surveys from patients who are already being prescribed off-label and experimental drugs. This is an important distinction between an observational and treatment study.
No ethics review board in Canada, to our knowledge, has approved the use of puberty blockers or cross-sex hormones for the treatment of youth who would like to align their physical characteristics with their gender identity or to avert health concerns such as depression and suicidality that can occur in youth experiencing gender dysphoria.
The Role of Puberty Blockers
Many Trans-supportive websites suggest that the intent behind starting a regime of puberty blockers is to “hit the pause button”.
The TransCare BC provincial health services authority says “Delaying puberty gives you more time to explore your gender identity, before changes happen to your body that can’t be reversed.” They describe the effects of taking puberty blockers as follows:
If you were assigned male at birth, puberty blockers will stop or limit:
- growth of facial and body hair
- deepening of the voice
- broadening of the shoulders
- growth of adam’s apple
- growth of gonads (testes) and erectile tissue (penis)
If you were assigned female at birth, puberty blockers will stop or limit:
- breast tissue development
- broadening of the hips
- monthly bleeding
In both cases, puberty blockers will temporarily stop or limit:
- growth in height
- development of sex drive
- impulsive, rebellious, irritable or risk-taking behaviour
- accumulation of calcium in the bones
- fertility
Are puberty blockers safe?
The Trans Youth Can! website states that “these treatments are generally considered safe” however there is little research or evidence to support this claim.
The Endocrine Society Clinical Practice Guideline published in November, 2017 is also very short on evidence. Of the Endocrine Society guidelines, Dr William Malone, Endocrinologist notes:
The document relies on a single, uncontrolled study to justify treating gender dysphoric youth with puberty blockers and cross-sex hormones. The sections detailing puberty blockade and cross-sex hormones provide “suggestions”, not recommendations, based on “low quality” evidence.
Dr William Malone, Endocrinologist
The major problem with the single study used to justify gender-affirming hormone treatment is the absence of a control group. A number of factors could have affected the results, including participation in intensive monthly therapy sessions. As a result, useful conclusions from the study are limited.
Longer term studies, such as the UK’s 5 year Tavistock puberty blocker treatment study showed that psychological well-being does not necessarily improve after a regime of puberty blockers and cross-sex hormones. Normal development of bone density was also discovered to be severely impaired.
Michael Biggs, Professor of Sociology at Oxford University published a detailed review of the study results and contributed several analyses to the UK group Transgender Trend. These analyses pointed out inconsistencies in the findings being reported, alarming omissions and obfuscating presentations of the data. This analysis eventually resulted in the UK study of puberty blockers being pulled into the spotlight of a parliamentary ordered review.
The UK Health Research Authority published the results of their investigation in October of this year and stated:
It would have reduced confusion if the purpose of the treatment had been described as being offered specifically to children demonstrating a strong and persistent gender identity dysphoria at an early stage in puberty, such that the suppression of puberty would allow subsequent cross-sex hormone treatment without the need to surgically reverse or otherwise mask the unwanted physical effects of puberty in the birth gender.
UK Health Research Authority
This was an admission that the initial goal of evaluating the impact on persistence and desistence rates that was outlined in the study protocol was never fulfilled. In fact, all participants in the study who started on puberty blockers proceeded to cross-sex hormones.
How should Puberty Blocker treatment be described?
A very important recommendation of the UK Health Research Authority is with respect to the characterization of the use of puberty blockers. Patients and their parents who are making a decision to begin an irreversible medical pathway that results in infertility and the requirement of a lifetime of medicalization need accurate information. “Researchers and clinical staff should consider carefully the terms that they use in describing treatments e.g. avoid referring to puberty suppression as providing a “breathing space”, to avoid risk of misunderstanding.”, the HRA stated in their review.
It is our position that there is a significant level of misinformation being communicated by Canadian health authorities with respect to the intent and safety of medical interventions such as puberty blockers. Almost any information source on the use of puberty blockers in Canada from provincial health systems or organizations such as Rainbow Health Ontario states that puberty blockers are used to create a “pause” and are safe and fully reversible.
The effects of puberty blockers are fully reversible. If you decide to stop taking them, your body will go through puberty just the way it would have if you had not taken puberty blockers at all.
TransCare BC Provincial Health Authority
Available evidence indicates that children who begin puberty suppression are very likely to continue with cross-sex hormones and will require invasive surgical procedures to provide artificial means of sexual functioning. Infertility is guaranteed.
TransCare BC, as an example, refers to these risks with statements such as “If you start taking puberty blockers early in puberty you might not be able to have the vaginoplasty surgery that is most commonly used in Canada.” This is because a section of colon will need to be removed and used to construct a vagina.
Independent Research Is Needed
The Trans Youth Can! Study will provide an interesting perspective on youth undertaking gender-affirming hormone therapy. We see a number of issues with the study design, however.
First of all, participation in the study is optional and the results gathered should be characterized as self-reported. These factors alone may lead to a biased sample of youth who are firmly committed for whatever reason to pursuing hormone therapy. Secondly, a study period of 2 years is hardly enough time to track “outcomes” or the stability of gender identity over time; one of the goals stated by the Trans Youth Can! Reserach team at the CPATH conference in 2017.
Further, the research team is closely affiliated with Trans Pulse Canada, an organization that requires it’s board of directors to be majority trans. Dr Greta Bauer is the research director of both the Trans Pulse survey of the trans community in Canada which has been credited with changing legislation and policy in Canada and this Trans Youth Can! study of youth referred for puberty blockers and hormone treatment.
The Trans Youth Can! initiative, therefore, is clearly not an independent medical study of youth who are being given life-changing hormone treatments. This intellectual conflict of interest is concerning to us as the trans-community-based research initiatives of Trans Pulse Canada are heavily invested in lobbying for changes in Canadian policy and legislation and would benefit from data on self-reported health outcomes of trans youth, particularly from a biased sample that can provide evidence to support their political goals.
Implications for Government Healthcare
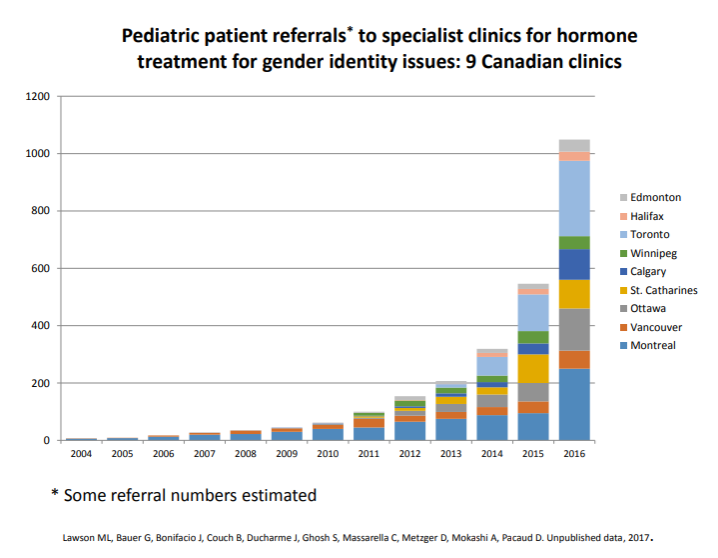
The recent adoption of the WPATH gender-affirming care model by the provincial health authorities in Canada coincides with an exponentially increasing number of children being socially and medically transitioned.
This model of care requires a burden of treatment that is entirely experimental and demands accurate information, if not a solid evidence base. We call on Canadian health authorities to require long-term studies of clinical outcomes for all youth who participate in gender-affirming hormone treatments. These studies must follow youth through adult care as well to determine whether their long-term health and well-being is improved.
The Trans Youth Can! Study is an impressive initiative as the largest international study of youth referred for hormone treatment at 300 patients but this number represents less than 15% of Canadian youth who are being referred for gender-affirming hormone treatment every 2 years based on the data provided by the gender clinics. Off label or not, the medical community prescribing puberty suppression and cross-sex hormone therapy under the recently adopted gender affirming care model has a responsibility to track and report the medical outcomes from this experimental form of treatment.
It is unconscionable to continue funneling more and more children into treatment with puberty suppression and cross-sex hormones unless there is a solid evidence base to prove the long-term health outcomes of this high-risk approach are significantly better than other treatment options.
In addition, until such time that puberty suppressing pharmacological treatments have been approved for the treatment of youth who wish to align their physical characteristics with their gender identity we demand that the Canadian government require accurate information that clearly identifies this treatment is experimental as it requires the off-label usage of drugs that will result in infertility and other compromising physiological effects when used over an unknown period of time. The type of information that is currently being promoted to the public is a serious misrepresentation of the experimental nature of puberty blockers and cross-sex hormone treatments targeted at a youth population.
We will be sending a letter to the Canadian provincial health authorities with these observations and requests.
Feature image source and data source: Trans Youth Can!